

Spontaneous Reactions:Spontaneous reactions are favourable.
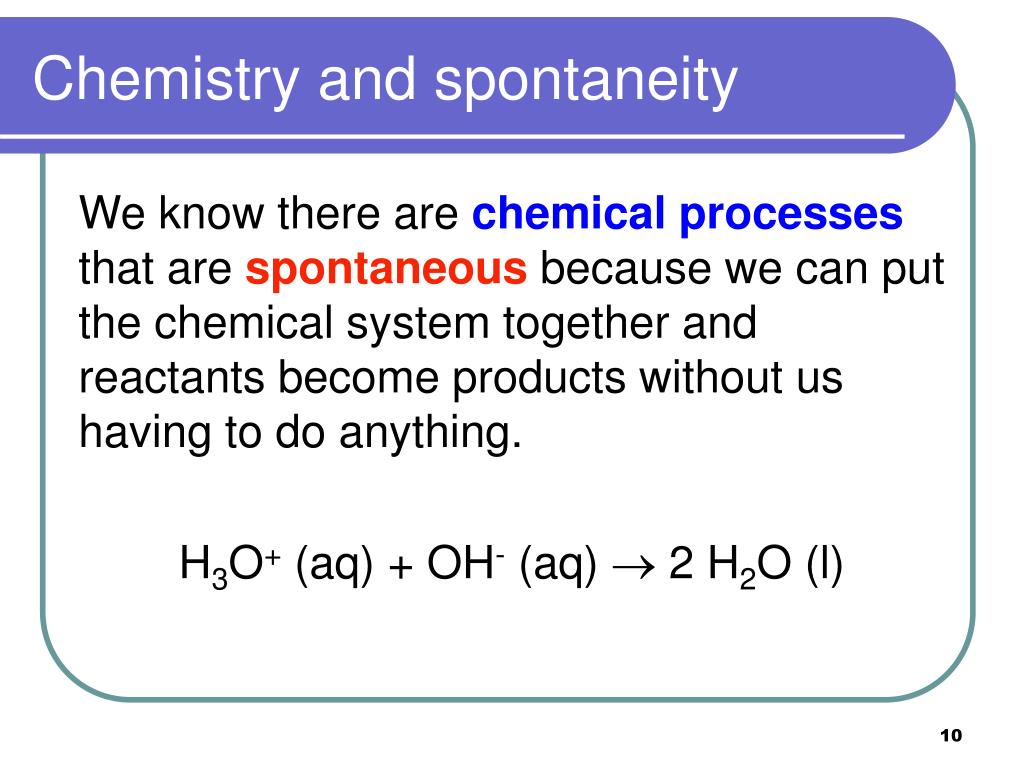
Nonspontaneous Reactions:Nonspontaneous reactions refer to the chemical reactions that require an energy input to proceed. Spontaneous Reactions:Spontaneous reactions refer to the chemical reactions that occur without being driven by an outside force. Both spontaneous and nonspontaneous reactions obey the three laws of thermodynamics.ĭifference Between Spontaneous and Nonspontaneous Reactions Definition.Both spontaneous and nonspontaneous reactions occur in a system with defined boundaries.Both spontaneous and nonspontaneous reactions occur in the environment.Similarities Between Spontaneous and Nonspontaneous Reactions But, at very high temperatures such as when lightning, this reaction is favourable. This means the reactants of the chemical reaction, i.e., nitrogen and oxygen gases, are more stable than the product: nitrogen monoxide. At the normal atmospheric pressure and temperature, this reaction is unfavourable. The reaction between atmospheric nitrogen and oxygen is an example of a nonspontaneous reaction.
#SPONTANEOUS AND NON SPONTANEOUS REACTION FREE#
As spontaneous reactions fulfil the above two conditions, they occur without inside intervention.įigure 2: The Change of the Gibbs Free Energy/Time When the occurrence of a chemical reaction decreases the enthalpy and increases the entropy of the system, it is considered as a favourable reaction. It describes the randomness and disorder of molecules. Entropy is the other thermodynamic property that accounts for the system’s thermal energy per unit temperature. Enthalpy is a thermodynamic property of a system that is the sum of the internal energy added to the product of the pressure and the volume of the system. The two driving forces of a chemical reaction are enthalpy and entropy. Spontaneous reactions refer to the chemical reactions that occur without being driven by an outside force. Key Terms: Endergonic Reactions, Entropy, Exergonic Reactions, Gibbs Free Energy, Nonspontaneous Reactions, Spontaneous Reactions What is the Difference Between Spontaneous and Nonspontaneous Reactions What are the Similarities Between Spontaneous and Nonspontaneous ReactionsĤ. The main difference between spontaneous and nonspontaneous reactions is that spontaneous reactions release free energy from the system, making it more stable whereas nonspontaneous reactions increase the total energy of the system. On that account, spontaneous reactions are exergonic while nonspontaneous reactions are endergonic. In nonspontaneous reactions, the change in the Gibbs free energy is positive. Therefore, these reactions release energy to the surroundings in the form of heat.
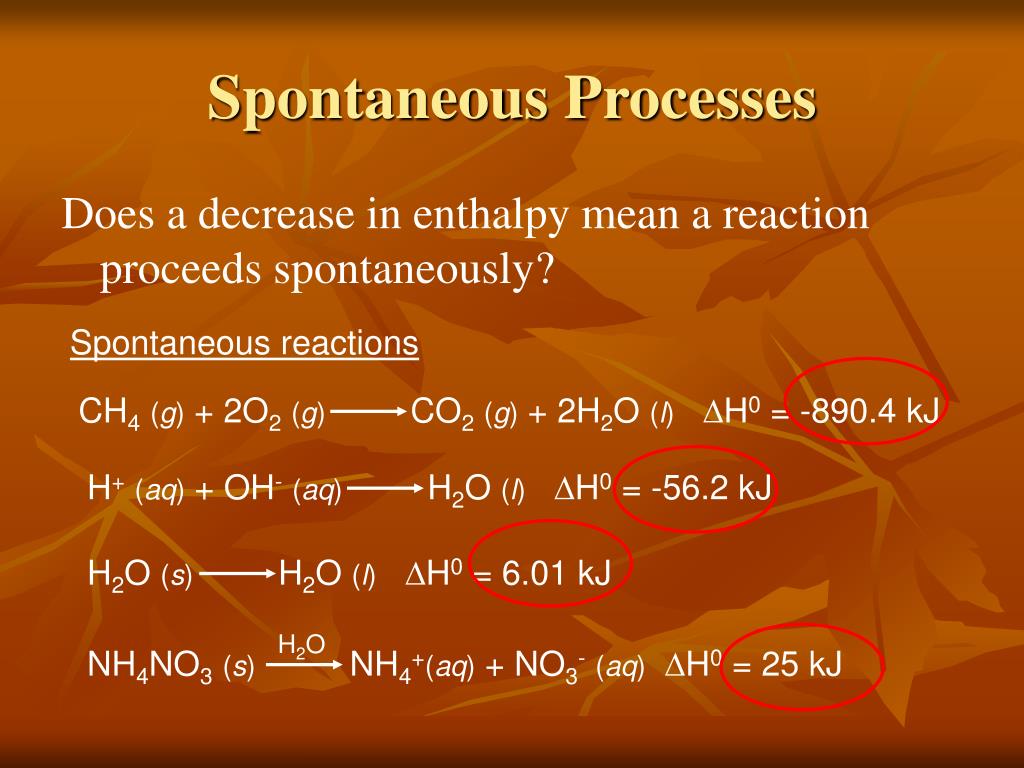
The change in the Gibbs free energy is negative for spontaneous reactions. However, energy should be provided for the nonspontaneous reactions to proceed. Spontaneous reactions take place on their own under a given set of conditions. Spontaneous and nonspontaneous reactions are the two types of chemical reactions that can occur in the environment. Main Difference – Spontaneous vs Nonspontaneous Reactions


 0 kommentar(er)
0 kommentar(er)
